Isosafrole
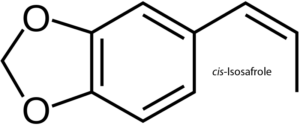
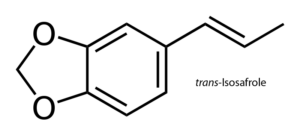
Isosafrole molecule structure is related to phenylpropene another aromatic organic chemical. Existing as two geometric isomers called cis-isosafrole and trans-isosafrole. Isosafrole can be obtained by the process of isomerizing the plant oil of safrole a slight yellowish oilish liquid removed from the root bark or the fruit sassafras plants. Because of it's use in ecstasy the US requires permits to buy or sell in any needed quantities. Using vacuum reflux by taking KOH as a catalyst and performing under vacuum with no solvent is usually preferable for many reasons. One reason the vacuum assist in removing the water at (~15%) in the KOH. The lower temperature of the reflux prevents the excessive side reactions. It is possible to reused the residue of KOH in the distillation flask to isomerise another safrole batch. The reflux (=reaction) temp is limited by the vacuum which also assist in removing the water which is formed by the reaction of the KOH and safrole. As such the higher the temp is the faster this reaction ends. As a rule the vacuum being half translates to a lower boiling point of about 10°C. A product boiling at a 200°C at 1000 mbar (athmospheric) will boil at around 190° at 500mbar, 180° at 250mbar, 170°C at 120 mbar, 160°C at 60 mbar, 150°C at 30mbar etc.. This is a good estimate on the boiling points. Magnetic stirring is mandatory but avoid with more than 1L safrole. Remember to use distilled safrole as when there is too much water available the results will be a failure. Distill iso with column and try to collect in the mid 80% then recycle the first and last 10%. The KOH residue in the distillation flask can be reused to isomerise another batch of safrole. The vacuum limits reflux (=reaction) temp and also helps in removing water formed by the reaction of KOH with the safrole. The higher the temperature, the faster this reaction will be over. As a rule of thumb, half the vacuum means lowering the boiling point about 10°C. A substance boiling at 200°C at 1000mbar (athmospheric) will boil at 190° at 500mbar, 180° at 250mbar, 170°C at 120 mbar, 160°C at 60 mbar, 150°C at 30mbar and so on. This is not exact, but can be used to estimate the expected boiling points roughly. Magnetic stirring is absolutely necessary. Avoid stirring this with more than 1L safrole, strong bumping was experienced with bigger amounts. Use distilled safrole! When there's too much water present, reaction fails. Distill the iso with column, preferrably collecting the middle 80%. Recycle the first and last 10%. There is difficulty in regulating the vacuum and to keep it constant. At full vacuum use a diaphragm pump. Do not use a rotary vane oil pump as it's ineffective as it pulls more vacuum than it should. Chemical formula is C10H10O2 Molar mass is 162.19 g·mol−1 Melting point is 8.2 °C (46.8 °F; 281.3 K) trans -21.5 °C, cis Boiling point is 253 °C (487 °F; 526 K) trans 77–79 °C, cis @ 3.5 mmHg Density is 1.1206 g/cm3, trans 1.1182 g/cm3, cis